“We should have some presence of mind, and how about we just set this aside and simply continue regularity, please,” one parent said to a series of praise.
Challenge Getting Younger Americans Vaccinated
Board Chairperson Wei Ueberschaer was met with boisterous booing as she communicated her mistake with the choice.
“I’m really miserable that face veils have transformed from a defensive methodology to a policy-centered issue,” Ueberschaer said.
The locale’s choice came that very day that Gov. Ron DeSantis marked a bill and two chief orders restricting Covid-19 limitations that provinces and urban areas can force.
The Florida Department of Education said the request taking effect right now “just affects city and area governments, and doesn’t affect school regions and individual schools.”
Miami-Dade County Public Schools, the fourth biggest area in the nation, said it would keep its cover order set up. Palm Beach County schools are additionally keeping its cover order. On the off chance that an understudy doesn’t consent to the region’s approach, the understudy might be relegated to far off picking up, as per the area’s Covid-19 conventions.
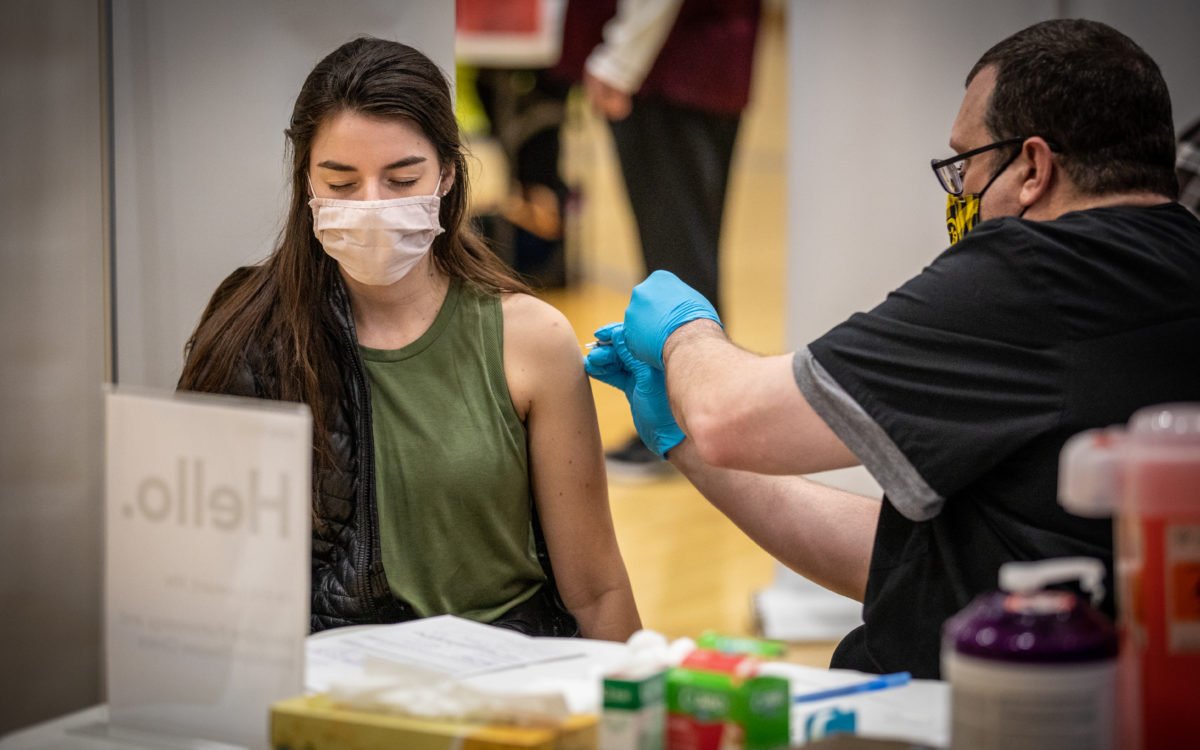
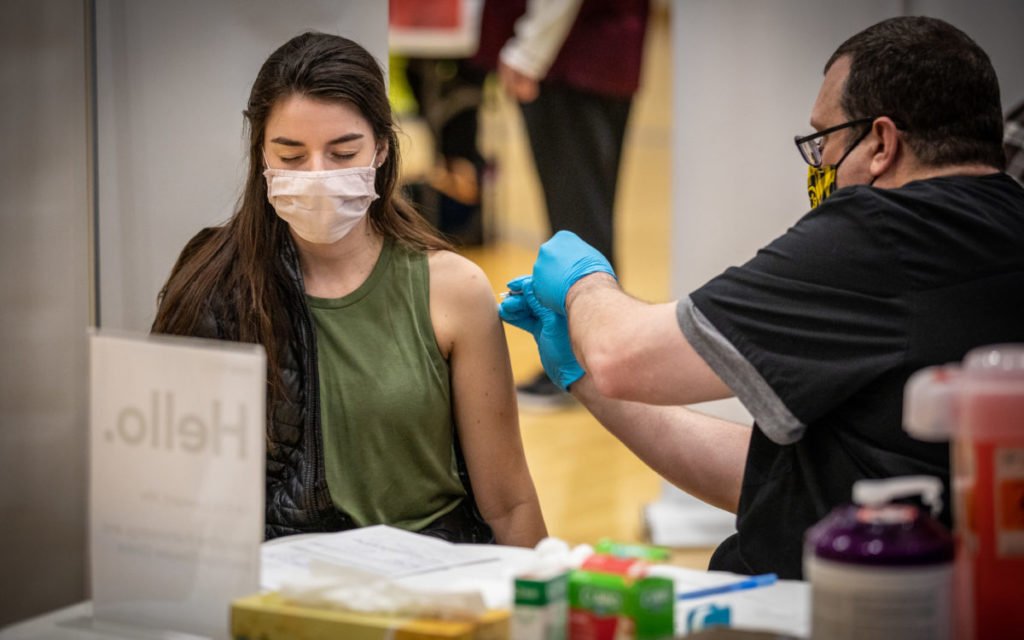
The US immunization rate has eased back, and specialists say the nation is presently at the critical step of its vaccination endeavors: arriving at Americans who aren’t as anxious to get a shot or still have difficulties with access.
Over 44% of the complete US populace has gotten at any rate one antibody portion, and almost 32% is completely immunized CDC information shows.
Among US grown-ups, over 56% have gotten at any rate one shot, the information shows, and over 40.5% are completely inoculated.
When the US can immunize over 70% of its grown-ups, it might at long last have the option to see a similarity to routineness, Jha said.
“Case numbers will dive. We may not be at crowd invulnerability; we’ll see little episodes to a great extent, yet life will start to truly return to ordinary,” he said.
Yet, consider the possibility that we don’t arrive.
“That is an issue,” Jha said. “We will be left with managing this for quite a while.”
“Assuming we simply don’t inoculate, clearly something we’ve known is we get large flare-ups, you can get more variations,” he said. “It will be difficult to do those huge social occasions, indoor shows, open-air ball games, this stuff will get a whole lot harder on the off chance that we don’t gain more headway on inoculations,” he added.
What could almost certainly occur, one master said, is networks that have a lower inoculation inclusion will keep on seeing high transmission of the infection, while in different pieces of the country with more immunizations, case rates will be a lot lower.
“In this nation, there’s a genuine separation around immunization,” previous acting CDC chief Dr. Richard Besser told CNN. “Individuals tend to live among individuals with comparable convictions.”
A significant approval could come one week from now
The one interconnecting piece specialists say is missing is getting kids vaccinated.
In any case, there’s uplifting news on that front.
A central government official told CNN on Monday that the FDA is ready to approve Pfizer/BioNTech’s Covid-19 immunization in kids and teenagers 12 to 15 years of age by right on time one week from now.
Pfizer has applied for crisis use approval. The FDA, which is presently assessing information put together by Pfizer, should revise the crisis use approval for the antibody, yet the cycle ought to be clear, the authority said.
A gathering of counsels to the CDC will plan a gathering for after any FDA choice to stretch out the EUA to new age gatherings and encourage the CDC to suggest the utilization of the antibody in 12 to long-term olds.
Walensky will, at that point, need to choose whether the office will suggest the antibody’s utilization in the new age bunch.
“That will quickly add a huge number of more individuals qualified for immunization. I bet a great deal of those children will get immunized,” Jha told CNN. “That will have a major effect too regarding developing populace insusceptibility.”
Pfizer and Moderna are trying their immunizations in youngsters as youthful as a half year old and hope to ask the FDA for EUAs covering babies and kids not long from now.
Pfizer has likewise submitted data to the FDA that may permit its immunization to be put away at standard fridge temperatures, its CEO said Tuesday. Such a change could help improve the appropriation of immunization.
Bourla said the organization submitted new information to the FDA on Friday. It might “soon” have a crisis use approval to permit standard refrigeration for as long as about a month.
Pfizer’s antibody is presently approved to be put away at ultracold temperatures, between – 80 and – 60 degrees Celsius, or in chilly stockpiling, between – 25 and – 15 degrees Celsius, for a limit of about fourteen days, which can confound circulation of the immunization.
New information could permit the immunization to be put away at standard temperatures, somewhere in the range of 2 and 8 degrees Celsius, for as long as about a month, as indicated by Bourla.